Percent yield calculator
Understanding and Using the Percent Yield Calculator
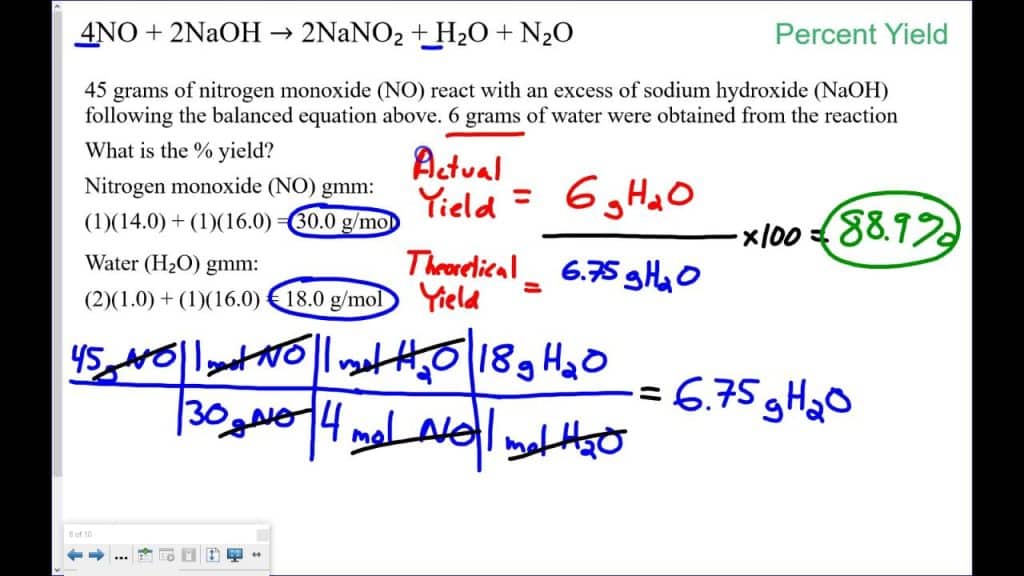
In the world of chemistry and chemical reactions, one of the most important concepts is the percent yield. It measures the efficiency of a chemical reaction by comparing the actual yield to the theoretical yield.
Calculating the percent yield is a crucial step in laboratory work and industrial processes, as it helps chemists assess the success of their reactions and optimize production processes.
To make these calculations more accessible and accurate, scientists and students often rely on the percent yield calculator. In this article, we will explore what percent yield is, why it’s important, and how to use a percent yield calculator effectively.
Understanding Percent Yield
Before delving into the specifics of a percent yield calculator, it’s essential to understand what percent yield represents. In chemistry, reactions are rarely perfect, and there are numerous factors that can lead to less-than-ideal outcomes. Percent yield provides insight into how much of the desired product is actually obtained compared to what could theoretically be produced under ideal conditions.
The formula for calculating percent yield is relatively straightforward:
Percent Yield (%) = (Actual Yield / Theoretical Yield) × 100
Where:
- Actual Yield is the amount of product obtained from a real chemical reaction.
- Theoretical Yield is the amount of product that should be obtained under ideal conditions, as predicted by stoichiometry and the balanced chemical equation.
In essence, percent yield quantifies the efficiency of a chemical reaction, indicating how successful the reaction was in converting reactants into products.
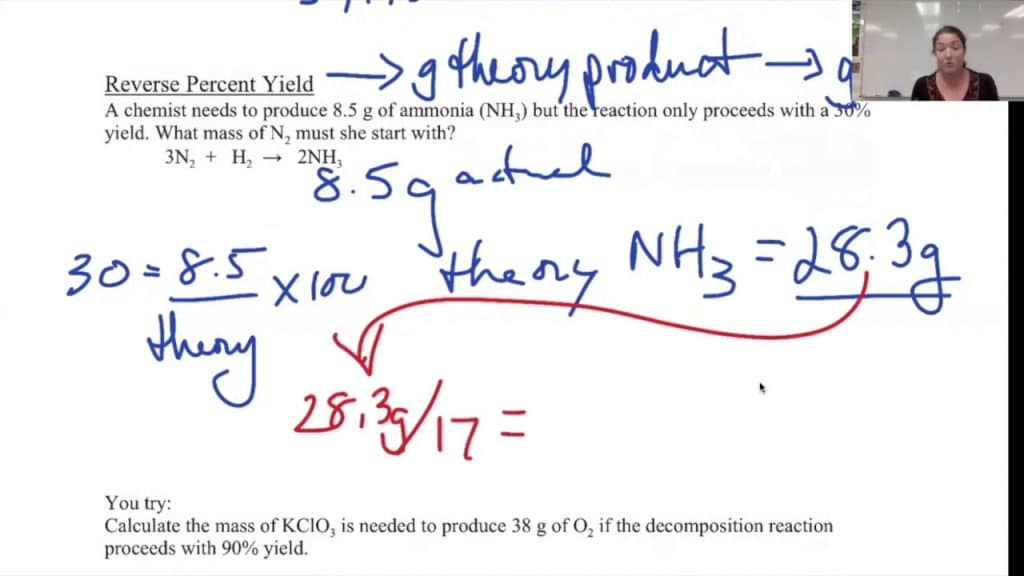
The Importance of Percent Yield
Percent yield is a fundamental concept in chemistry with significant practical implications. Here’s why it’s so important:
Quality Control: In industries that rely on chemical processes, percent yield is a crucial parameter for quality control. It helps ensure that the production process is efficient and that the desired products are obtained in the expected quantities.
Cost Efficiency: By calculating percent yield, companies can minimize waste and optimize resource utilization. This is particularly important when dealing with expensive or limited resources.
Safety: Understanding the efficiency of a chemical reaction can also enhance safety. Reactions that don’t proceed as expected may produce hazardous byproducts or lead to dangerous conditions.
Research and Development: In research labs, percent yield is used to evaluate the success of experimental procedures. Researchers can adjust reaction conditions and parameters based on percent yield data to achieve better results.

Using a Percent Yield Calculator
Now that we understand the significance of percent yield, let’s explore how to use a percent yield calculator effectively.
- Gather Information:
- You’ll need to know the balanced chemical equation for the reaction you’re interested in.
- Measure the actual amount of product obtained during the experiment.
- Calculate Theoretical Yield:
- Use stoichiometry to calculate the theoretical yield. This involves converting the moles of reactants to moles of products using the mole ratio from the balanced equation.
- Convert moles to grams if necessary.
- Input Values:
- Open the percent yield calculator of your choice (many online tools are available).
- Enter the actual yield and the theoretical yield.
- Calculate Percent Yield:
- The calculator will use the formula mentioned earlier to calculate the percent yield.
- The result will be a percentage representing the efficiency of your reaction.
- Interpret the Result:
- A percent yield of 100% indicates a perfect reaction where all reactants were converted to products.
- Percent yields above 100% may occur if there is more actual product obtained than theoretically predicted. This can happen due to impurities or side reactions.
- Percent yields below 100% suggest that the reaction did not proceed as efficiently as expected, likely due to factors such as incomplete reactions, impurities, or losses during handling.
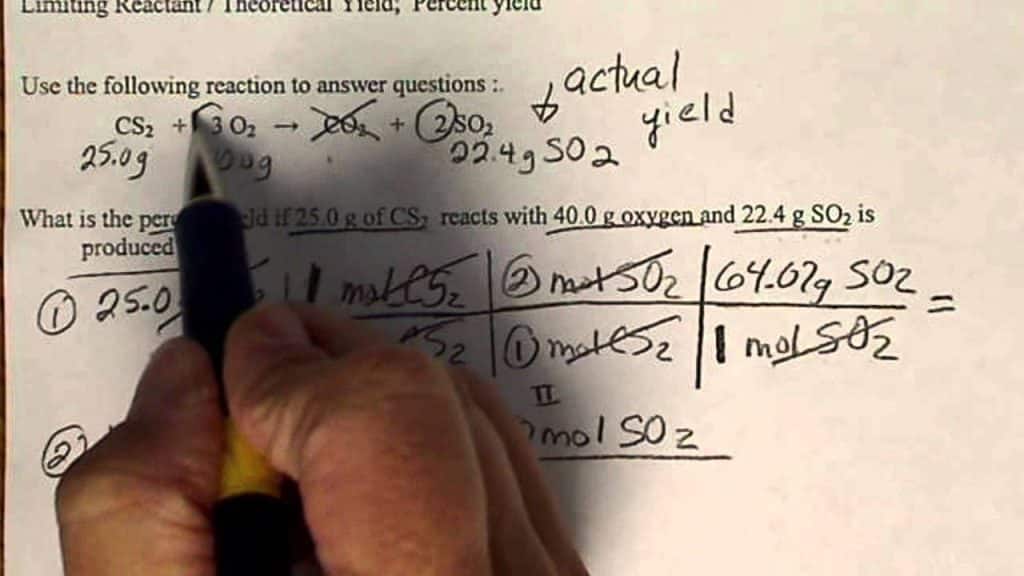
Conclusion
In the world of chemistry, the percent yield calculator is a valuable tool for assessing the efficiency of chemical reactions. It helps chemists and scientists in various fields ensure the quality of their work, optimize processes, and make informed decisions.
By comparing actual and theoretical yields, the percent yield provides valuable insights into the success of a reaction. Whether you’re a student learning about stoichiometry or a professional in a chemical industry, mastering the use of the percent yield calculator is essential for accurate and efficient work in the field of chemistry.
